Why is Adding Fluoride to Drinking Water Controversial?
Adding fluoride to drinking water is controversial due to a combination of health, ethical, and scientific concerns. Proponents argue that fluoride significantly reduces dental cavities and benefits public health, particularly in communities with limited access to dental care. However, opponents raise health concerns, citing studies that suggest excessive fluoride exposure can lead to adverse effects such as dental and skeletal fluorosis, potential bone cancer, and other health issues. Ethically, some individuals argue that water fluoridation is a form of mass medication without informed consent, infringing on personal choice. Additionally, scientific debates continue over the optimal fluoride levels that balance dental benefits with minimizing health risks, leading to differing regulations and recommendations worldwide. This combination of health risks, ethical considerations, and scientific uncertainty fuels the ongoing controversy surrounding water fluoridation.
Health Effects of Fluoride in Drinking Water
For a variety of health reasons, many people are looking for ways to remove fluoride from their drinking water or seeking out bottled water without fluoride. While water fluoridation is a widespread water treatment practice in the United States and a few other places, opponents of fluoride, and many preventative health professionals, now believe that fluoride reacts with every enzyme and hormone in the human body and that it causes bones to harden prematurely, possibly leading to bone cancer, especially in young boys.
The late Dr. John Yiamouyiannis, author of the book “Fluoride: The Aging Factor,” strongly believed and wrote that ingested fluoride was not only a significant “aging factor” but that it did very little to lessen tooth cavities as well. He fought for and was very successful in having fluoridation discontinued in many parts of the world. This revolt against fluoride has caused many people to avoid fluoride by distilling their water for consumptive purposes. Even though it’s still very controversial, fluoride remains part of a huge $15 billion per year fluoro-chemical sales industry in the United States each year.
Remove Fluoride from Drinking Water
Activated Carbon Filters and Sediment Filters: Standard pitcher filters will do nothing to reduce or remove fluoride. One exception may be carbon char filters.
Alumina Filters: These can remove up to 99% of fluoride from water but they add aluminum, which could be problematic for most people.
KDF Filters: These do not remove fluoride. KDF stands for Kinetic Degradation Fluxion and consists of high-grade copper/zinc media.
Reverse Osmosis Systems: These can remove fluoride by 85-92% when new but lose effectiveness over time. They also waste many gallons of water to produce a gallon of treated water, making them less environmentally friendly.
Ion Exchange Filters: These can remove fluoride (a negative ion) almost 99% until the anion resin is exhausted.
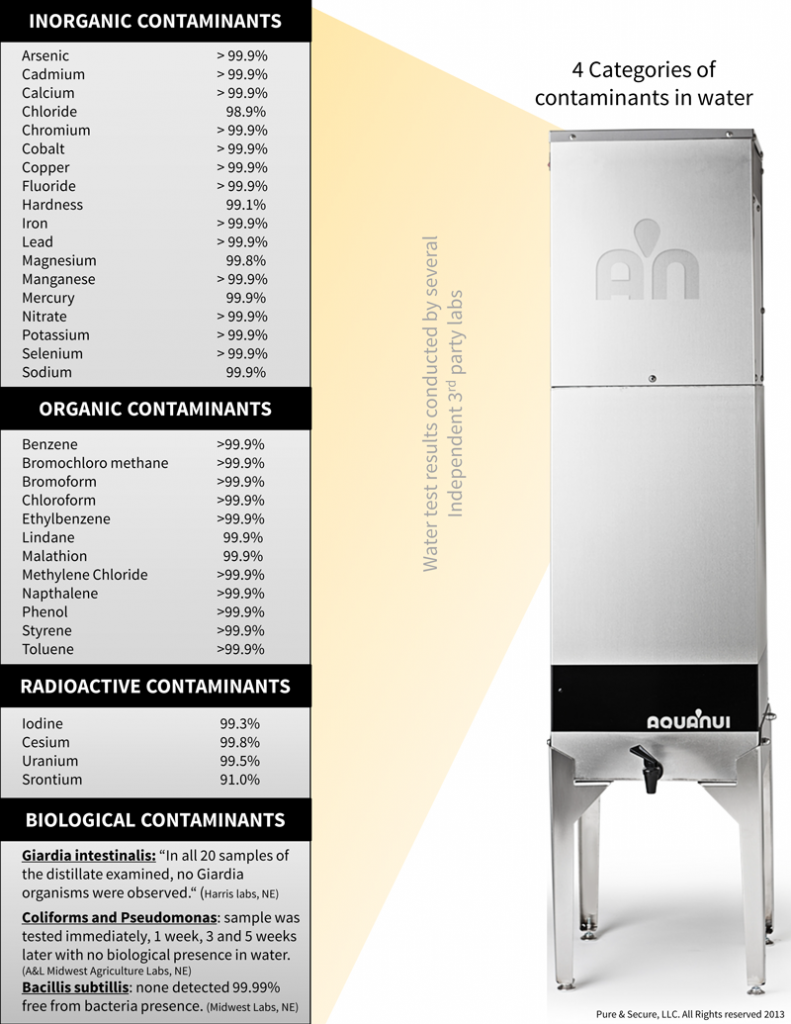
Water Distillation Systems: Distillation is the best way to remove fluoride from drinking water. Third-party lab testing reports show that the removal rate of fluoride by an AquaNui distiller is greater than 99%. While water filters that remove fluoride are an option, for long-term continuous removal, distillation is by far the most effective.
Do Water Filters Remove Fluoride? No. Common pitcher filters or carbon filters will not remove fluoride. Distillation is the best way to remove fluoride from drinking water.
EPA Primary Standard for Fluoride in Drinking Water
Federal and State regulations require that fluoride, which occurs naturally in some water supplies, not exceed a concentration of 4.0 mg/L in drinking water. This is a Primary Drinking Water Standard which means that it is Federally enforceable and Water Municipalities are subject to daily fines for each day they exceed the standard. Exposure to drinking water levels above 4.0 mg/L for many years may result in some cases of crippling skeletal fluorosis, which is a serious bone disorder.
EPA Secondary Standard for Fluoride in Drinking Water
Primary Standard: Federal and state regulations require that fluoride, which occurs naturally in some water supplies, not exceed a concentration of 4.0 mg/L in drinking water. This is a primary drinking water standard, meaning it is federally enforceable, and water municipalities are subject to daily fines for each day they exceed the standard. Exposure to drinking water levels above 4.0 mg/L for many years may result in crippling skeletal fluorosis, a serious bone disorder.
Secondary Standard: Federal and state regulations also require a water system to notify the public when monitoring indicates that the fluoride in drinking water exceeds 2.0 mg/L. Because this is a secondary standard, it is not federally enforceable. This is intended to alert families about dental problems that might affect children under nine years of age. Fluoride in children’s drinking water at levels of approximately 1 mg/L is believed to reduce the number of dental cavities. However, some children exposed to levels of fluoride greater than about 2.0 mg/L may develop dental fluorosis. Dental fluorosis, in its moderate and severe forms, results in brown staining and/or pitting of the permanent teeth.
Important Notice: The U.S. Environmental Protection Agency recently announced that it has reevaluated the current science on fluoride. The EPA will rely on these new assessments to review the existing maximum level of fluoride allowed in drinking water and determine whether its drinking water regulations for fluoride should be revised. The EPA will review the drinking water standards to ensure they continue to protect against the unwanted effects of excessive exposure. EPA’s examination of the fluoride drinking water public health goal and enforceable standard will be based on this new science, along with other information such as analytical methods and treatment feasibility.
Commercial Uses for Fluoride
Owing to the great expense of refining pure fluorine, most commercial applications use fluorine compounds. About half of the mined fluorite is used in making steel. The rest of the fluorite is converted into corrosive hydrogen fluoride (hydrofluoric acid) on its way to becoming various organic fluorides or cryolite, which plays a key role in aluminum refining. Organic fluorides have very high chemical and thermal stability; their major uses are as refrigerants, electrical insulation, cookware, and PTFE (Teflon).
Pharmaceuticals such as atorvastatin and fluoxetine also contain both fluorine and the fluoride ion. Fluoride is thought to inhibit dental cavities, which is why it is added to most kinds of toothpaste today, albeit with a warning for misuse. However, there are many types of toothpaste found in health food stores or online that do not contain fluoride.
Fluorine is a chemical element with the symbol F and atomic number 9. It has nine protons and usually ten neutrons in its nucleus, with seven electrons in its outermost shell. Fluorine is not used in water treatment, as it would have fatal results. It has a melting point of -219.62 degrees Celsius at one atmosphere, a boiling point of -188.14 degrees Celsius at one atmosphere, and a specific gravity of 1.108, making it a little heavier than pure water. It has a valence of 1, meaning it needs one electron to complete its outer shell of electrons.
Fluoride is a negative ion formed from the element fluorine. Its chemical symbol is F-1. This is the result of fluorine taking one electron from a metal or electron donor. It is used in water treatment and is called fluoridation.
How Abundant is Fluoride?
Fluorine is the thirteenth most common element in the Earth’s crust at 600-700 parts per million by mass. Elemental fluorine would readily react with water vapor in the Earth’s atmosphere, so there is no elemental fluorine in the air. Fluorine is found only in combined mineral forms, with fluorite, cryolite, and fluorapatite being the most industrially significant. When compared to other elements, fluorine ranks 24th in abundance.
Historical Context: Fluorite, the mineral that gave fluorine its name, was first described in 1529. At that time, fluorite was added to metal ores to lower their melting points for smelting purposes. The Latin verb “fluo” meaning “flow” gave the mineral its name. Fluorine was proposed as an element in 1810 but proved very difficult and dangerous to separate from its compounds. Several early experimenters sustained serious injuries or even died from their attempts.
In 1886, a French chemist named Henri Moissan isolated elemental fluorine relatively safely by using the process of low-temperature electrolysis for the first time. This process is still used today for the modern production of fluorine. Industrial production of fluorine gas for uranium enrichment began during the Manhattan Project and remains its largest industrial application.
Fluorocarbon gases are considered greenhouse gases with a global warming potential 100-20,000 times that of carbon dioxide. They tend to persist in the environment due to the strength of the carbon-fluorine chemical bond. Fluorine has no known metabolic role in humans or other mammals, though a few plants synthesize organo-fluorine poisons to deter herbivores.
Should I Drink Water With Fluoride?
Every consumer will need to decide for themselves whether or not they want to ingest fluoride with their tap water. While fluoride may have benefits for dental health according to many, there are legitimate concerns about its potential adverse health effects. The debate over fluoride in drinking water is complex, involving scientific, regulatory, and public health considerations. For those seeking to avoid to remove fluoride from drinking water, there is nothing more effective and environmentally friendly than using a distillation system like an AquaNui Water Distiller.